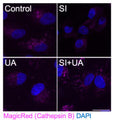
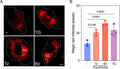
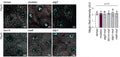
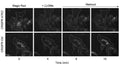
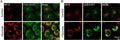
- Prepare samples and controls
- Reconstitute Magic Red by adding DMSO.
- Dilute Magic Red 1:10 by adding diH2O.
- Add 20 µL Magic Red to each sample (~ 500 µL aliquot of cultured cells).
- Incubate while protected from light.
- Watch color start to develop within 15 minutes.
- If desired, label with additional stains, such as Hoechst, DAPI, or an antibody.
- If desired, fix cells.
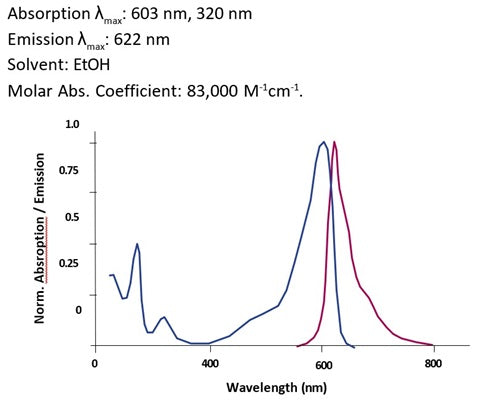
- Analyze with a fluorescence microscope or fluorescence plate reader. Magic Red has an optimal excitation at 592 nm and emission at 628 nm.
If working with adherent cells, please see the manual for additional protocols.
Product Specific References
| PMID | Publication |
| 39757940 | Song, C, et al. 2025. Nonreceptor tyrosine kinase ABL1 regulates lysosomal acidification by phosphorylating the ATP6V1B2 subunit of the vacuolar-type H+-ATPase. Autophagy, 45677. |
| 39689715 | Xu, Y, et al. 2024. The cGAS-STING pathway activates transcription factor TFEB to stimulate lysosome biogenesis and pathogen clearance. Immunity, . |
| 39715749 | El Fissi, N, et al. 2024. Preventing excessive autophagy protects from the pathology of mtDNA mutations in Drosophila melanogaster. Nature Communications, 10719. |
| 39675068 | Song, C, et al. 2024. Sarcopenic obesity is attenuated by E-syt1 inhibition via improving skeletal muscle mitochondrial function. Redox Biology, 103467. |
| 39454993 | Tam, E, et al. 2024. Autophagy deficiency exacerbated hypoxia-reoxygenation induced inflammation and cell death via a mitochondrial DNA/STING/IRF3 pathway. Life Sciences, 123173. |
| 39303907 | Neto, M.V., et al. 2024. PHOTOPROTECTIVE MELANIN IS MAINTAINED WITHIN KERATINOCYTES IN STORAGE LYSOSOMES. The Journal of Investigative Dermatology, . |
| 39057917 | Killips, B, et al. 2024. Coxiella burnetii inhibits nuclear translocation of TFEB, the master transcription factor for lysosomal biogenesis. Journal of bacteriology, e0015024. |
| 38993141 | Long, Z, et al. 2024. Enhanced autophagic clearance of amyloid-β via HDAC6-mediated V-ATPase assembly and lysosomal acidification protects against Alzheimer's disease in vitro and in vivo. Neural regeneration research, . |
| 38980070 | White, MD, et al. 2024. Selective host autophagy is induced during the intracellular parasite Toxoplasma gondii infection controlling amino acid levels. mSphere, e0036924. |
| 38901330 | Daughtry, A, et al. 2024. Development and Diagnostic Potential of a Novel Bartonella henselae-Specific Immunoglobulin. Diagnostic microbiology and infectious disease, 116381. |
| 38947172 | Cerda-Troncoso, C, et al. 2024. Chemo-small extracellular vesicles released in cisplatin-resistance ovarian cancer cells are regulated by the lysosomal function. Journal of Extracellular Biology, e157. |
| 38870060 | Bienvenu, A, et al. 2024. The multifunction Coxiella effector Vice stimulates macropinocytosis and interferes with the ESCRT machinery. PNAS: USA, e2315481121. |
| 38890703 | Jiménez-Loygorri, JI, et al. 2024. Urolithin A promotes p62-dependent lysophagy to prevent acute retinal neurodegeneration. Molecular neurodegeneration, 49. |
| 38885848 | Gao, AYL, et al. 2024. RIT2 regulates autophagy lysosomal pathway induction and protects against α-synuclein pathology in a cellular model of Parkinson's disease. Neurobiology of disease, 106568. |
| 38849544 | Sun, H, et al. 2024. Polystyrene nanoparticles trigger aberrant condensation of TDP-43 and amyotrophic lateral sclerosis-like symptoms. Nature nanotechnology, 0. |
| 38840105 | Hua, KF, et al. 2024. Cinnamaldehyde inhibits the NLRP3 inflammasome by preserving mitochondrial integrity and augmenting autophagy in Shigella sonnei-infected macrophages. Journal of inflammation (London, England), 18. |
| 38837642 | Mächtel, R, et al. 2024. Late-onset Krabbe disease presenting as spastic paraplegia - implications of GCase and CTSB/D. Annals of clinical and translational neurology, 0. |
| 38795919 | You, H.J., et al. 2024. Disruption of early embryonic development in mice by polymethylmethacrylate nanoplastics in an oxidative stress mechanism. Chemosphere, 142407. |
| 38578235 | Mulligan, RJ, et al. 2024. Collapse of late endosomal pH elicits a rapid Rab7 response via the V-ATPase and RILP. Journal of Cell Science, . |
| 38525600 | Konietzny, A, et al. 2024. Efficient axonal transport of endolysosomes relies on the balanced ratio of microtubule tyrosination and detyrosination. Journal of cell science. |
| 38388414 | Du, J., et al. 2024. Regulation of NCOA4-mediated iron recycling ameliorates paraquat-induced lung injury by inhibiting ferroptosis. Cell communication and signaling : CCS, 146. |
| 38446621 | Sava, I, et al. 2024. Reversible assembly and disassembly of V-ATPase during the lysosome regeneration cycle. Molecular Biology of the Cell, ar63. |
| 38348092 | Ebstrup, ML, et al. 2023. Annexin A7 mediates lysosome repair independently of ESCRT-III. Frontiers in Cell and Developmental Biology, 1211498. |
| 38177283 | Yan, R., et al. 2024. Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion. Nature immunology. |
| 38130933 | Lee, J., et al. 2023. Particulate matter 10 induces oxidative stress and apoptosis in rhesus macaques skin fibroblast. PeerJ, e16589. |
| 37936170 | Wu, A.Y., et al. 2023. Spatiotemporal roles of AMPK in PARP-1- and autophagy-dependent retinal pigment epithelial cell death caused by UVA. Journal of biomedical science, 91. |
| 37981210 | Kim, Y., et al. 2023. A Surge of Cytosolic Calcium Dysregulates Lysosomal Function and Impairs Autophagy Flux during Cupric Chloride-Induced Neuronal Death. The Journal of biological chemistry, 105479. |
| 38009916 | Wang, Z., et al. 2023. The role of lysosomes as intermediates in betacoronavirus PHEV egress from nerve cells. Journal of virology, e0133823. |
| 37741453 | Quan, J.H., et al. 2023. Toxoplasma gondii induces pyroptosis in human placental trophoblast and amniotic cells by inducing ROS production and activation of cathepsin B and NLRP1/NLRP3/NLRC4/AIM2 inflammasome. The American journal of pathology, 00362-0. |
| 36707427 | Serra-Vinardell, J., et al. 2023. LYST deficiency impairs autophagic lysosome reformation in neurons and alters lysosome number and size. Cellular and molecular life sciences : CMLS, 53. |
| 36746928 | Marchand, B., et al. 2023. Gemcitabine promotes autophagy and lysosomal function through ERK- and TFEB-dependent mechanisms. Cell death discovery, 45. |
| 36814381 | Mahmood, A., et al. 2023. Exosome secretion kinetics are controlled by temperature. Biophysical journal. |
| 36914626 | Rowland, L.A., et al. 2023. De novo lipogenesis fuels adipocyte autophagosome and lysosome membrane dynamics. Nature communications, 1362. |
| 37098348 | Wang, Z., et al. 2023. Cellular proteins act as surfactants to control the interfacial behavior and function of biological condensates. Developmental cell. |
| 37056248 | Chaiamarit, T., et al. 2023. Fluorescence Assays for Real-Time Tracking of Cell Surface Protein Internalization and Endosomal Sorting in Axons of Primary Mouse Hippocampal Neurons. Bio-protocol, e4651. |
| 37213076 | Shelke, G.V., et al. 2023. Inhibition of endolysosome fusion increases exosome secretion. The Journal of cell biology. |
| 37269887 | Bellese, G., et al. 2023. Neratinib is a TFEB and TFE3 activator that potentiates autophagy and unbalances energy metabolism in ERBB2+ breast cancer cells. Biochemical pharmacology, 115633. |
| 37279824 | Tobias, T., et al. 2023. In vitro immune and redox response induced by cationic cellulose-based nanomaterials.. Toxicology in Vitro, 105616. |
| 37390818 | Zhang, J., et al. 2023. Lysosomal LAMP proteins regulate lysosomal pH by direct inhibition of the TMEM175 channel. Molecular cell. |
| 37284455 | An, J., et al. 2023. Nicotine exacerbates atherosclerosis and plaque instability via NLRP3 inflammasome activation in vascular smooth muscle cells. Theranostics, 2825-2842. |
| 37211762 | Wang, Y., et al. 2023. Polyphyllin D punctures hypertrophic lysosomes to reverse drug resistance of hepatocellular carcinoma by targeting acid sphingomyelinase. Molecular therapy : the journal of the American Society of Gene Therapy. |
| 37375535 | Zhang, C., et al. 2023. Bis-Benzylisoquinoline Alkaloids Inhibit Porcine Epidemic Diarrhea Virus by Disrupting Virus Entry. Pathogens (Basel, Switzerland). |
| 37416869 | Alhaider, A., et al. 2023. Insulin-like growth factor-1 improves in vitro meiotic resumption of dromedary camel (Camelus Dromedarius) oocytes. Animal Reproduction, e20220105. |
| 37452023 | Berquez, M., et al. 2023. Lysosomal cystine export regulates mTORC1 signaling to guide kidney epithelial cell fate specialization. Nature communications, 3994. |
| 37625588 | Noda, K., et al. 2023. Characterization of Rab32- and Rab38-positive lysosome-related organelles in osteoclasts and macrophages. The Journal of biological chemistry, 105191. |
| 35194165 | Wu, CI., et al. 2022. APP and DYRK1A regulate axonal and synaptic vesicle protein networks and mediate Alzheimer's pathology in trisomy 21 neurons. Molecular psychiatry. |
| 35121108 | Capuozzo, A., et al. 2022. Fluoxetine ameliorates mucopolysaccharidosis type IIIA. Molecular therapy : the journal of the American Society of Gene Therapy. |
| 35222364 | Santos, S.A.C.S., et al. 2022. P2X7 Receptor Triggers Lysosomal Leakage Through Calcium Mobilization in a Mechanism Dependent on Pannexin-1 Hemichannels. Frontiers in Immunology, 752105. |
| 35319746 | Murakawa, T., et al. 2022. A Drosophila toolkit for HA-tagged proteins unveils a block in autophagy flux in the last instar larval fat body. Development (Cambridge, England). |
| 35309917 | Vargas, G., et al. 2022. Negative Modulation of Macroautophagy by Stabilized HERPUD1 is Counteracted by an Increased ER-Lysosomal Network With Impact in Drug-Induced Stress Cell Survival. Frontiers in cell and developmental biology, 743287. |
| 35269509 | Huang, P., et al. 2022. Lysosomal ATP Transporter SLC17A9 Controls Cell Viability via Regulating Cathepsin D. Cells. |
| 36186127 | Lin, X., et al. 2022. Folic acid Ameliorates the Declining Quality of Sodium Fluoride-Exposed Mouse Oocytes through the Sirt1/Sod2 Pathway. Aging and Disease. |
| 35435672 | Li, Y., et al. 2022. Tumor Microenvironment-Responsive Yolk-Shell NaCl@Virus-Inspired Tetrasulfide-Organosilica for Ion-Interference Therapy via Osmolarity Surge and Oxidative Stress Amplification. ACS nano. |
| 35489582 | Wooten, J., et al. 2022. Dibenzyl trisulfide induces caspase-independent death and lysosomal membrane permeabilization of triple-negative breast cancer cells. Fitoterapia, 105203. |
| 35599014 | Zhong, W., et al. 2022. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging cell, e13622. |
| 35750034 | Hu, M., et al. 2022. Parkinson's disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes. Cell, 2292-2308.e20. |
| 37396505 | Tanno, T., et al. 2022. A novel aptamer-based small RNA delivery platform and its application to cancer therapy. Genes & Diseases. |
| 35771772 | Oe, Y., et al. 2022. PACSIN1 is indispensable for amphisome-lysosome fusion during basal autophagy and subsets of selective autophagy. PLoS genetics, e1010264. |
| 35977928 | Ratto, E., et al. 2022. Direct control of lysosomal catabolic activity by mTORC1 through regulation of V-ATPase assembly. Nature communications, 4848. |
| 36044857 | Akter, E., et al. 2022. Non-degradable autophagic vacuoles are indispensable for cell competition. Cell reports, 111292. |
| 36055242 | Fernandez, M.A., et al. 2022. Loss of endosomal exchanger NHE6 leads to pathological changes in tau in human neurons. Stem cell reports. |
| 36044568 | Iyer, H., et al. 2022. A lysosomal regulatory circuit essential for the development and function of microglia. Science advances, eabp8321. |
| 36195598 | Hao, W., et al. 2022. Autophagy induction promoted by m6A reader YTHDF3 through translation upregulation of FOXO3 mRNA. Nature communications, 5845. |
| 36180832 | Li, Y., et al. 2022. Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62-Keap1-NRF2 pathway. Cellular & molecular biology letters, 81. |
| 36197338 | Hiragi, S., et al. 2022. TBC1D18 is a Rab5-GAP that coordinates endosome maturation together with Mon1. The Journal of cell biology. |
| 36512240 | Mulligan, R.J., et al. 2022. Endosomal Transport to Lysosomes and the Trans-Golgi Network in Neurons and Other Cells: Visualizing Maturational Flux. Methods in molecular biology (Clifton, N.J.), 595-618. |
| 33414397 | Zhao, T., et al. 2021. Autophagy impairment as a key feature for acetaminophen-induced ototoxicity. Cell death & disease, 3. |
| 33502918 | Browning, C.L., et al. 2021. Manganese dioxide nanosheets induce mitochondrial toxicity in fish gill epithelial cells. Nanotoxicology, 44944. |
| 33386512 | Honma, Y., et al. 2021. Correlation of hepatitis C virus-mediated endoplasmic reticulum stress with autophagic flux impairment and hepatocarcinogenesis. Medical molecular morphology. |
| 33422265 | Miao, G., et al. 2020. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Developmental cell. |
Question: When you dilute the stock concentration 1/10 in H2O and aliquot is for future use, how long can this be stored? I see that the stock concentration in DMSO can be stored for 6 months.
Answer: Once the stock solution is diluted 1:10 in diH2O we recommend using within 15 minutes to prevent hydrolysis of the Magic Red substrate. Non-specific hydrolysis would lead to fluorescence not associated with enzymatic cleavage of the substrate. We do not recommend attempting to aliquot or store the diH2O-diluted substrate solution for future use. Yes, that’s correct. The DMSO-reconstituted Magic Red stock can be stored frozen for up to 6 months protected from light and thawed more than twice during that time.