Anti-Capsaicin receptor (VR1/TrpV1) Antibody (BS397)
Our Anti-Capsaicin receptor (TrpV1) mouse monoclonal primary antibody detects guinea pig (predicted), mouse, and rat Capsaicin receptor (TrpV1), and is IgG. It is validated for use in FC, WB.
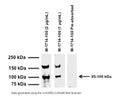
B: Western blot of TrpV1 in rat PC12 cell lysates (80 µg/lane). M-1714-100 detects TrpV1 protein at 95-100 kDa. SDS-PAGE: denatured and reduced; Transfer: Tris-Glycine buffer; Membrane: nitrocellulose (0.45 µm); Blocking: 5% skim milk in TBST, 1 hour at RT; Primary antibody: overnight at 4°C (2 µg/mL); Secondary antibody: anti-mouse-HRP (1/6000) 2 hours at RT; Detection: Chemiluminiscence.
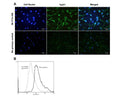
C: Immunohistochemical staining of TrpV1 in mouse dorsal root ganglia. Immunoreactivity was visualized with anti-mouse-Cy3 conjugate (red). Magnification: 20x. Courtesy P. Vilimas, Flinders University Adelaide.
D: Western blot (denatured and reduced) of TrpV1 in cell lysates of forskolin and NGF stimulated 50B11 hybrid mouse x rat DRG cell lines and NGF-stimulated PC12 cells (10 µg/lane). M-1714-100 detects monomeric TrpV1 protein at 95-100 kDa. Primary antibody: 1 µg/mL (4°C overnight). Detection: Chemiluminiscence. Courtesy Dr. D. Matusica, Flinders University.
SKU: M-1714-100
Product Details
IHC: 1-10 µg/mL
ICC: 1-2 µg/mL
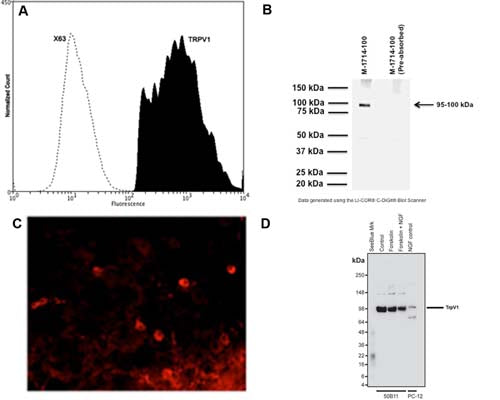
Flow Cytometry: 2 ug/10^6 cells.
Western blotting: 0.5-2 µg/mL , SDS-PAGE on Bis-Tris gel 4-12%, 5% beta-mercaptoethanol, primary antibody O/N incubation in 5% skim milk/TBST. Secondary is anti-mouse-HRP, 1/6000 dilution, 2h at room temperature. Blot developed on Li-Cor C-DiGit Scanner.
IHC: Frozen or PEG embedded tissues tested (PEG embedding, see Klosen P et al (1993) J Histochem Cytochem. 41(3):455-63). Conditions tested: 1-10 µg/mL in PBS, 48 hours, followed by detection via directly conjugated fluorescent anti-mouse secondary.
Immunohistochemistry Protocol for Product Image
4% PFA perfused, formalin fume-fixed DRG tissues: Adult male rat was perfusion-fixed transcardially with 4% paraformaldehyde. The lumbar dorsal root ganglia were isolated and embedded into egg yolk medium, which was solidified in formalin fume. 30-µm-thick floated sections were prepared with a Vibratome. For pretreatment, the sections were thoroughly rinsed in 0.1M PBS (pH 7.6) and then incubated with a mixture of 0.5% H2O2 and 0.2% Triton X-100 for 30 min. Then, antigen retrieval was performed with 0.1M citrate buffer (pH=6.0) at 80°C for 30 min. (Please note that without this step, we did not obtain any labeling). Then, the TRPV1 signal was detected using a 1:5000 dilution of the primary antibody in 2% normal horse serum (NHS) in PBS (16 h; RT), followed by a 1:500 dilution of the biotinylated secondary antibody (2 h in 2% NHS/PBS; RT) and then, the ABC Elite reagent (Vector; 1:1000 in Tris buffer; pH 7.6). The signal was visualized with Ni-DAB chromogen. The immunostained sections were rinsed in Tris buffer, mounted on microscope slides, air dried, counter-stained with cresyl violet, dehydrated and coverslipped with DPX mounting medium.
[Immunohistochemistry protocol generously provided by Dr. Szabolcs Takacs & Dr. Erik Hrabovszky, Institute of Experimental Medicine, Budapest, Hungary].
Antibody not yet tested on paraffin-embedded sections. Other immunohistochemistry methods have not yet been tested but are expected to be reactive under correct conditions. Antigen recovery is suggested; the epitope is internal cytoplasmic and could be covered by internal proteins during fixation.
ICC: 4% formaldehyde fixed cells tested; requires permeabilization step as antigen epitope is intracellular. Suggested primary antibody concentration: 1-2 µg/mL.
"