Function of the Blocker/Stabilizer
- Within a vast majority of immunoassay formats, the sample-interactive surfaces on which antigen or antibody is adsorbed or covalently bound, must always be treated with some type of blocking agent. The short-term goal being to cover up, or block, vacant regions of the sample-interactive-surfaces not presently occupied by antigen or antibody molecular structures. In the longer term, this action should prevent down-stream assay reactants from non-specifically adhering to those vacant spots on the assay surfaces. By restricting their presence, non-specific binding (NSB) signal can be kept to a minimum (Figure 1).
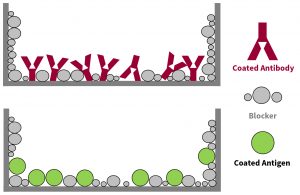
Figure 1. Schematic showing blocker molecules covering up, or blocking, vacant regions of the plate well surface not occupied by coated antibodies or antigens.
- Of equal importance, adsorption of blocking agents to the sample-interactive-surfaces, which also includes the adsorbed antigen and antibody components, should concurrently confer an elevated level of molecular antigen or antibody stability (Figure 2). This attribute is essential if there are any aspirations of long term dry-state storage.
Blocker/Stabilizer Search Process
Before venturing further into this blocker/stabilizer selection discussion, it is paramount to emphasize that there is no such thing as a universally optimal (for all immunoassay situations) blocker/stabilizer formulation. Stating this a different way, no commercial or in-house formulated blocker/stabilizer can make the claim that it is the superior choice for all immunoassay sample types and assay configurations. When the assay mechanism is reliant upon an antibody/antigen interaction, each scenario is unique. This makes it highly likely that your first selection of blocker/stabilizer product may not in fact be the only or best choice for your unique assay conditions, antibody/analyte interaction dynamics, or signal generation mechanism.
Matching Blocker/Stabilizers with Different ELISA Scenarios
Antigen-Down ELISA formats
Antigen-Down (AD) ELISA formats are aptly named for the fact that a targeting antigen is pre-adsorbed onto the inner surface of 96-well plate wells. Traditionally, AD ELISA formats were the go-to testing strategy for detection and quantitation of serological humoral responses to specific pathogen or autoimmune events. With the conceptual simplicity of the AD serological testing format comes the challenge of keeping non-specific IgG binding event to a minimum. AD ELISA plate-well surface associated, non-specific binding targets are two-fold. There is the obvious need to cover up/block all unoccupied (by antigen) surface regions on plate-well surfaces, as well as to block any sticky molecular structures on the adsorbed antigen components. Adequately blocking smaller molecular weight antigen coated plates can present some challenges. Use of the common-place BSA (molecular weight 68 kDa) based blocker could run the risk of obscuring the surface availability of the smaller molecular weight antigen epitopes. When smaller < 10 kDa protein and oligo-peptide antigens are the adsorbed target, deliberate selection of a smaller molecular weight blocker or synthetic based blocker may be helpful.
Antibody-Sandwich ELISA formats
Antibody-Sandwich (AS) ELISA formats get their name from the fact that an antigen capturing IgG is pre-adsorbed onto the inner surface of the 96-well plate wells. Traditionally, AS ELISA testing formats were the gold-standard for establishing normal physiological concentration ranges for multitudes of clinically relevant serological effector molecules. The AS ELISA was developed to enable the sensitive detection and quantitation of biologically relevant target molecules (usually proteinaceous in nature). Once again, the task of adequately blocking critical sample-interactive-surfaces within an AS ELISA can be a more complex undertaking than one might initially expect. Blocker additive components must target both the unoccupied (by antigen-capture IgG) plate-well surface regions, as well as the molecular “sticky” regions of the plate-well adsorbed IgG components. With respect to blocking strategies, the obvious goal once again is to always cover up/block all exposed polystyrene surface regions as well as block any unusually “sticky” molecular structure regions on the plate adsorbed IgG.
Blocker Choice Examples for Different ELISA Scenarios
- Developing an AD ELISA to monitor serological levels of IgM with specificity for a particular protein that is localized within joint tissues.
Suggestion: For most initial antigen-down ELISA development scenarios, the use of ICT’s Neptune Block Buffer product is a safe and likely optimal choice. Neptune Block Buffer contains a heterogeneous mixture of non-mammalian proteins that have proven over the years to minimize non-specific binding interactions within AD ELISA testing formats.
- Developing a routine AS ELISA intended for monitoring serological levels of an inflammation associated cytokine.
- Elevated non-specific background signal observed within an otherwise functional AS ELISA. Currently using an in-house BSA based blocker formulation after initially using an in-house casein driven blocker.
- Developing an AS ELISA. Want to enhance assay sensitivity through the use of an avidin-HRP plus biotin signal amplification protocol.

